Good to know:
Some basic knowledge about brain anatomy and neuroimaging is helpful to understand section 3 of the paper better; however, the section should also be understandable on its own - other important terms and concepts are explained within the paper.
Summarizing Statement
Prenatal alcohol exposure is a preventable cause of lifelong disability, leading to fetal alcohol spectrum disorder (FASD). Individuals with FASD often face lifelong challenges and may struggle to live independently. The disorder manifests in physical deformations and neurobehavioral impairments.
Children with FASD show difficulties in cognitive development, including impaired IQ, attention organization issues, and executive function challenges, negatively impacting their academic performance.
These children also experience higher rates of school dropout and behavioral problems, which may not solely stem from cognitive issues but also from social challenges and environmental factors.
Early diagnosis and intervention are crucial for supporting affected individuals, and further research should focus on their cognitive development and potential alleviation of FASD symptoms.
Cognitive Development of Children with Histories of Prenatal Alcohol Exposure

Introduction
In 1973, alcohol was identified to be a substance leading to irreversible damage to children when consumed by mothers during pregnancy (Mattson2019 Riley2011). Next to facial and bodily deformations, prenatal alcohol exposure (PAE) is also known to lead to cognitive impairments. This essay will provide on the state of research regarding the cognitive development of children with histories of prenatal alcohol exposure. PAE is a preventable cause of life-long disability that can lead to fetal alcohol spectrum disorder (FASD), which includes several different disorders (Kully-Martens2012 Riley2011). The exact prevalence of FASD is difficult to estimate since FASD often remains un- or misdiagnosed. Estimations in the US include anything between 2% and 5% of the population (Riley2011 Fan2016). Since PAE does not necessarily lead to FASD, the percentage of women who drank during pregnancy is presumably higher than the estimated number of children affected by FASD. This assumption is verified by the Centers for Disease Control and Prevention, which conducted a study with pregnant women in the US between 2006 and 2010. 7,6% of the participants had consumed alcohol during the prior 30 days (Fan2016 Moore2014).
Individuals with FASD face lifelong challenges due to the disorder and often do not manage to live independently. The increased demand for support from educational, social, and behavioral services, as well as the medical and justice systems, makes FASD expensive for the countries in question. In Canada, for example, 5.3 billion dollars are spent each year to support individuals with FASD (Kully-Martens2012 Moore2021 Riley2011).
To be able to properly support FASD individuals, FASD must be diagnosed as early as possible. In the past, the diagnostic criteria have focused on the often more obvious and characteristic physical deformations. However, there are forms of FASD that are characterized by neurobehavioral impairments only. Thus, the neurobehavioral profile is crucial in order to diagnose all forms of FASD correctly and make it possible to provide FASD individuals with the help they need (Mattson2019 Riley2011).
The exact diagnostic criteria for the different forms of FASD, as well as difficulties with establishing these criteria, will be discussed in Section 1. Section 2. gives an overview of the methodological approaches to investigate how children’s cognitive development after PAE is altered. While this section explains the general experimental design, specific study designs and tasks are addressed in the corresponding sections. In section 3., neurophysiological abnormalities are discussed, as well as mechanisms that lead to their development.
Sections 4 to 7 outline different aspects of cognitive development that are impaired in children with PAE, presumably as a consequence of the brain changes discussed in section 3. Section 4. takes a closer look at intelligence quotient (IQ), attention, and language development. Section 5. discusses executive function and, in more detail, spatial working memory. Section 6. gives an overview of the academic performance of children with PAE. Section 7. concludes the essay and gives an outlook on further open questions regarding the effects of PAE.
Section 1: Diagnosis
PAE can but need not necessarily lead to many impairments and disorders, summarised under the umbrella term “fetal alcohol spectrum disorder” (FASD) (Fan2016 Riley2011 Moore2014). The severity of the impairments depends on the amount of alcohol consumed, the gestational week during consumption, and the frequency of consumption. The more alcohol is consumed, the greater the danger of impairments becomes. Additionally, binge drinking (i.e., the compression of the consumed alcohol into a few hours rather than a larger time frame) is more damaging than continuous drinking. While drinking can be harmful at any stage of pregnancy, drinking is especially detrimental in critical phases of brain development occurring in earlier stages of pregnancy (Mattson2019). To date, however, no critical values have been found for how much PAE leads to FASD or if there are periods or alcohol amounts that are acceptable and safe during pregnancy (Riley2011 Fan2016).
Alcohol enters the mother’s bloodstream after consumption and can enter the fetal blood system as well. This way, it can reach the developing organs and tissue of the fetus. Brain development is affected the most, as brain abnormalities can occur. As a result, leading to neurobehavioral symptoms (Riley2011).
The most severe form of FASD is the fetal alcohol syndrome (FAS), followed by the partial fetal alcohol syndrome (PFAS). Milder forms include but are not limited to, alcohol-related neurodevelopmental disorder (ARND) and alcohol-related birth defects (ARBD) (Mattson2019 Riley2011). It is important to note that diagnostic criteria vary greatly between countries and papers as no unified diagnostic criteria exist (Mattson2019). Thus, the following diagnostic criteria should only be taken as a general guideline.
According to (Mattson2019), to be diagnosed with FAS, children need to show the following four symptoms: facial anomalies, physical growth deficiency, abnormal brain growth, and neurobehavioral impairments (including cognitive and/or behavioral impairments). Since FAS is the most severe form, children only need to display a subset of the symptoms to be diagnosed with another form of FASD. PFAS requires the presence of only two symptoms: facial anomalies and neurobehavioral impairments. If there is no documented alcohol exposure, either growth deficiency or abnormal brain growth is additionally needed for a diagnosis of PFAS. A diagnosis of ARBD requires a history of PAE and one or more physical abnormalities (Mattson2019).
Due to the characteristic facial and bodily deformations, FAS and PFAS (and, to a lesser extent, ARBD) are easy to diagnose, and earlier research focused on children diagnosed with one of the three syndromes. However, children with prenatal alcohol exposure (PAE) often show neurobehavioral impairments while lacking physical deformities. These children are diagnosed with ARND (Riley2011).
To be able to intervene early, an early diagnosis is necessary (Riley2011 Mattson2019). Diagnosis of ARND is especially difficult since the neurobehavioral impairments are not as characteristic as the physical impairments and can be easily confused with other syndromes. Additionally, there is a high overlap of FASD with other disorders, for example, attention deficit hyperactivity disorder (ADHD). The symptoms of ADHD are similar to the neurobehavioral FASD symptoms, and around 50% of FASD children were also diagnosed with ADHD, which might increase the probability of confusion of FASD with ADHD. Furthermore, due to the stigma surrounding drinking during pregnancy, drinking reports from mothers are hard to obtain, making it difficult to identify children at risk. In a sample of foster and adopted children, 80% of the children affected by FASD had never been diagnosed, while 6.4% were misdiagnosed (Mattson2019).
Since neurobehavioral impairments are present among many FASD individuals, researchers have started to focus on creating a clear neurobehavioral profile, which is distinct from other similar disorders, to facilitate the diagnosis of FASD (Mattson2019 Kully-Martens2012 Riley2011). Before outlining the profile, the following sections will concern methodological approaches and brain development.
Section 2: Methodology
To investigate neurobehavioral impairments, researchers compare children affected by PAE with a control group. Sometimes, the PAE group is split into subgroups according to the different syndromes, e.g., FAS, PFAS, ARND and non-syndromic children with PAE, since PAE does not have to result in FASD (Fan2016).
Since the effects of PAE can be observed throughout life, studies can be conducted with PAE individuals at any age. Some studies are cross-sectional, measuring different children at each time point (e.g., ten children of age 6, ten of age 7, and so on), as opposed to longitudinal, in which an individual is tested repeatedly at different time points, allowing for more precise tracking of cognitive development than in cross-sectional studies (Moore2021).
To be able to identify children with PAE, researchers need drinking reports. In the optimal case, these are obtained during pregnancy or directly after delivery since retrospective reports, for example, six years after pregnancy, are not as reliable anymore (Fan2016). The stigma associated with alcohol consumption during pregnancy makes it hard to obtain reports directly from the mothers. Thus, researchers also use birth records, reports from children, youth services’ documentation, as well as reports from other family members (Kully-Martens2012 Mattson2019).
Alcohol consumption can be measured in two different ways: average alcohol per day or week (e.g., on average one drink each day or 7 per week) or alcohol per occasion and how many occasions there are per week (e.g., six drinks per occasion at two times per week). The latter is referred to as “binge-drinking” (Fan2016). As stated in the introduction, critical levels of alcohol consumption leading to FASD are unknown. Researchers thus set this amount of alcohol to be in the PAE or control group. While the amount differs between studies, the critical values for the PAE lie between 2 – 14 drinks per week or more than 4 – 5 drinks per occasion at least once or twice per week (Moore2021 Fan2016 Howell2005).
Typical conditions to be recruited for the control group are no brain injury or neurological conditions in children, no or very little maternal drinking or smoking during pregnancy and no complications during pregnancy and birth. However, control and PAE group conditions vary between studies (Kully-Martens2012).
If the children come to the attention of the researchers via mothers’ drinking reports, diagnosis is often missing. If scholars want to differentiate between the different outcomes of PAE, children will be diagnosed with the help of professionals, e.g., psychologists, speech-language pathologists, occupational therapists, social workers, and development pediatricians (Fan2016 Kully-Martens2012).
Depending on which aspect of cognitive development is tested, researchers employ a range of standardized tests or tasks, some of which will be introduced in later sections.
Before discussing the so-far-established neurobehavioral profile associated with cognitive impairments, I want to point out brain alterations, which are assumed to be the neurobiological basis for the cognitive impairments observed in children with PAE.
Section 3: Neurological Alterations
To investigate neurological alterations, studies employ neuroimaging techniques, such as magnetic resonance imaging (MRI) or diffusion tensor imaging (DTI). DTI is an MRI-based method using water molecule diffusion and can be used to investigate brain anatomy and white matter pathways. The obtained images show that FASD is correlated with decreased general brain volume (Fan2016 Mattson2019 Moore2014). This can also lead to microcephaly, meaning an abnormally small head, which is a physical deformation often seen in FASD (Fan2016). Decreased general brain volume negatively correlates with PAE, meaning that more alcohol exposure leads to larger decreases in brain volume (Moore2014). More specifically, both white matter (Riley2011 Fan2016 Moore2014) as well as grey matter is reduced. This is concerning since decreased brain volume predicts cognitive abilities. For example, smaller left hippocampi correlate with poorer verbal learning skills and spatial memory as this region is known to be involved in consolidation and retrieval of memory (Mattson2019 Moore2014).
Other specific brain regions that are altered include the caudate nucleus, which is part of the basal ganglia. Its volume correlates with cognitive control, verbal learning, and recall skills (Mattson2019 Moore2014). Furthermore, PAE can lead to corpus callosum agenesis or displacement, which encompasses either the missing of the corpus callosum or its malformation. The corpus callosum is a white matter structure important for communication between the two hemispheres (Fan2016 Mattson2019 Riley2011 Moore2014). Other regions altered in PAE include the amygdala, the thalamus, and the cerebellum (Mattson2019 Fan2016).
Using DTI on the whole brain, (Fan2016) tried to specifically investigate white matter abnormalities caused by PAE. They found reductions in total white matter volume and, more specifically, in frontal, parietal, temporal lobes, and the cerebellum. Since the general brain volume is decreased in children with PAE, as discussed above, the authors corrected their finding of total white matter volume reductions for total brain volume. Afterward, many of these findings were not significant anymore, showing that decreased white matter volume results from decreased brain volume. The authors also found alterations in the cerebellar peduncles. The cerebellar peduncles are a white matter structure connecting the cerebellum to the brain stem. Alterations included poorer myelination and/or poorer axon density. Since white matter is generally responsible for fast signal transmission, impairments in white matter and white matter pathways may lead to slower information processing, which is prevalent in children with PAE (Fan2016).
Lastly, abnormal network connectivity has been observed in the insula, the basal ganglia, the cerebellum, and the amygdala resulting in altered brain activity in verbal learning, response inhibition, visual attention, and working memory (Mattson2019).
These brain alterations are the effects of disrupted development in the uterus across all stages of brain development due to alcohol exposure. Atypical brain development is caused by a variety of mechanisms, such as neuronal and glial migration errors, disrupted cell interactions, altered gene expressions, oxidative stress, and growth factor signaling disruptions (Mattson2019 Riley2011 Fan2016).
The list of altered brain regions given above touches upon the significant topics but is not exhaustive. For a detailed review, see (Moore2014). The wide range of altered brain regions implies that many different brain functions are affected. The following sections will discuss different aspects of cognitive development impaired in children with PAE.
Section 4: IQ, Attention and Language Development
This section gives an overview of the effect of PAE on IQ and attention. IQ is usually assessed using standardized IQ tests like the Wechsler Intelligence Scale for Children-III (Lee2004 Howell2005). According to (Mattson2019), a decreased IQ is a common effect of PAE, albeit not a diagnostic requirement. It correlates with impairment, as children with FAS, the most severe form of FASD, have lower IQ scores than children with PFAS, ARND or ARBD (Mattson2019 Fan2016). This might be because IQ correlates positively with brain size, and, as discussed in section 3, PAE negatively correlates with brain size (Moore2014).
As pointed out in section 1, FASD is highly correlated with ADHD, which has attention deficits as its main symptom. Thus, it is not surprising that difficulties with attention have also been observed in children with FASD. These include establishing, organizing, and sustaining attention. Problems in sustaining attention might be due to damage to the midbrain and brainstem (Mattson2019). Scores on attention tests were able to predict whether a child belonged to the PAE or the control group with an accuracy of 91,7%, highlighting that attention deficits are an essential symptom of FASD. Attention problems are persistent over time and do not correlate with IQ. Furthermore, they are specifically noticeable in the visual, rather than the auditory domain (Mattson2019 Lee2004 Aragón2008).
Children with PAE also have poorer language skills and demonstrate errors even in fundamental skills, such as articulation and grammar. While younger children have these global deficits, older children can catch up. However, difficulties remain, for example, with language syntax. Children with FAS, the most severe form of FASD, struggle additionally with word order, sentence combining, and grammatical comprehension (Mattson2019).
Section 5: Executive Function
This section discusses impairments that individuals with PAE face in executive function. According to (Mattson2019), “executive function broadly refers to the higher-order inter-related cognitive processes (e.g., working memory, problem-solving, planning, response inhibition) that are involved in goal-directed behavior” (Mattson2019). Impairments in executive function may be mediated by damage to frontal brain regions, which has been observed in children with FASD, as pointed out in section 3 (Kully-Martens2012). Furthermore, they may be an effect of a decreased IQ. However, impairments in executive function are more severe than the IQ deficit alone would predict (Connor2000). Children who were diagnosed with some form of FASD had impairments across the whole domain of executive function (Mattson2019). However, impairments in executive function do not seem to be worse in individuals with FAS than in other individuals on the PAE spectrum (Green2009 Connor2000).
The following section discusses different aspects of executive function, namely verbal fluency, response inhibition, problem-solving and planning, concept formation, working memory, and spatial working memory. Verbal fluency can be tested with a task like this: “List all words you can think of that start with the letter ‘E’ in one minute.” or “List all the animals you know in one minute.”. FASD children produce fewer words than children from control groups, thus showing that verbal fluency is affected in FASD (Mattson2019 Aragón2008).
Response inhibition is the ability to suppress one response in favor of another. This can be tested with tasks where children must press a button under certain conditions and not press it under different conditions. While FASD children were able to inhibit responses, they reacted more slowly than children from control groups in trials in which they did not have to inhibit (Mattson2019). Additionally, FASD individuals show increased prefrontal cortex activation during the inhibition trials, showing that they need greater cognitive resources to complete the task (Green2009).
Problem-solving and action-planning are important everyday life skills. To test for these abilities, researchers present children with a specific problem they must solve and rules they must follow. Researchers can measure the time or the number of moves children need to solve the problem, as well as observe their strategies. FASD children use less efficient strategies. Thus, they need more moves or take longer to develop a solution. This might be because they plan their actions for a shorter time than children from control groups. Contrary to non-FASD children, FASD children directly start with the task, indicating that they do not think of a strategy beforehand. Additionally, FASD children also violate given rules more often than control groups. These differences between FASD and control groups grow larger as problem difficulty increases (Mattson2019 Green2009).
To form concepts, children need to be able to understand relationships between different objects. FASD children perform worse when forming concepts of given objects. Furthermore, they struggle to verbalize the concepts they have formed. When identifying concepts, FASD children make more mistakes than control groups. Lastly, FASD children also do not listen as well to feedback from researchers during the experiment (Mattson2019). The challenges children face when forming concepts might be due to their limited cognitive flexibility, a quality which is required for this portion of the executive function (Mattson2019).
Following the definition from (Mattson2019), “Working memory is a storage system with limited capacity that temporarily holds active information necessary for a variety of tasks including learning, comprehension, and reasoning.” Some researchers argue that working memory impairments might be the core deficit of FASD, making its investigation crucial for the neurobehavioral profile (Kully-Martens2012). Similar to problem-solving, impairments in working memory become stronger as task difficulty increases. Frontoparietal regions, impaired due to PAE (see section 3), are the assumed neurological basis for working memory. This essay focuses on spatial working memory, a part of working memory also impaired in FASD (Mattson2019).
Studies show that the difference in spatial working memory observed between PAE and control groups disappears after correcting for visual-spatial skills. Thus, visual-spatial skills, which generally are impaired in PAE individuals, are assumed to be the reason for spatial memory difficulties. The impairment might be due to atypical development and different activation patterns in frontal-parietal and temporal brain regions involved in this ability (Mattson2019 Moore2021).
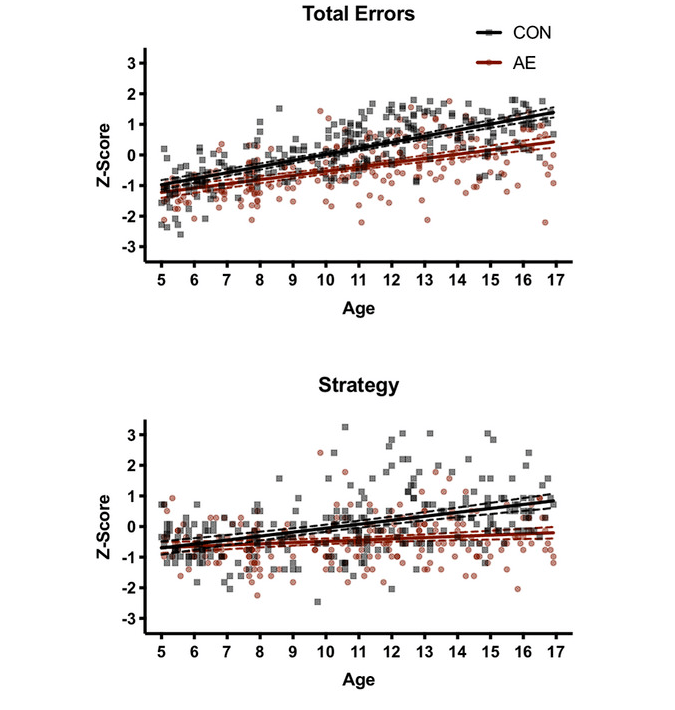
A study by (Moore2021) used a cross-sectional design to investigate spatial working memory in children with PAE. Children between the ages of 5 and 17 years old were divided into two groups: a PAE group and a group of typically developing children.
The critical values for PAE were four drinks per occasion at least once a week or exceeding thirteen drinks per week during pregnancy. The critical values for the control group were never more than one drink per week and never more than two drinks per occasion during pregnancy.
To assess spatial working memory, Moore et al. used the CANTAB Spatial Working Memory Subtest. During this test, participants see several boxes on a touchscreen. Under some of these boxes, a token is hidden, and participants must find it. Clicking on each box reveals whether it contains a token or not. The number of boxes on the screen goes up to eight boxes. Moore et al. measured z-scores of the errors of participants (e.g., how often a box already visited was clicked on again) and strategy (e.g., if children started with a different box for each trial or had a clear order in which they visited boxes) (Moore2021 Green2009).
As can be seen in figure 1, there was a difference between the groups. For both groups, overall performance increased between the ages of 5 and 17, showing that spatial working memory is improved during cognitive development. However, while younger children from both groups performed similarly, differences between groups became larger as children aged. FASD individuals made more errors and also had a worse strategy. The authors conclude that the development of spatial working memory is slower in PAE children than in control groups, making them fall behind (Moore2021). Similar results were also found in a study by (Green2009) that tested children with the CANTAB Spatial Working Memory Subtest. FASD children also made more errors and had worse strategy scores.
Section 6: Academic Performance
All the impairments listed so far suggest that children with PAE or FASD have difficulties in academic performance. FASD children have, in fact, higher rates of dropping out and being suspended or expelled from schools compared to control groups. These problems arise both from cognitive, as well as social issues. On the one hand, FASD children have difficulties in mathematical skills even after correcting for IQ and perform poorly on spelling and reading tasks. The former may be due to spatial processing deficits and a consequence of working memory impairments, as discussed in Section 5. On the other hand, FASD children struggle to fit into social environments like schools, as they have low socialization skills and often display aggressive or inappropriate sexual behavior (Mattson2019). Lastly, FASD is also correlated with criminal activity, alcohol, and drug problems, amplifying these problems (Mattson2019).
However, a study by (Howell2005) compared academic behavior in alcohol-exposed adolescents with a non-alcohol-exposed control group that, contrary to other control groups, shared the same low socioeconomic status. The researchers noted no significant difference between the groups in rates of dropping out, being suspended or expelled from schools or causing problems in school. This implies that some of the issues in schools PAE individuals face may be a consequence of environmental factors such as their socioeconomic status rather than their alcohol exposure (Howell2005).
Section 7: Summary and Outlook
This essay gave an overview of impairments PAE children face in cognitive development. These include an impaired IQ, problems sustaining and organizing attention, problems in executive function – most notably in working memory, including spatial working memory, and language development, leading to poorer academic performance. To support PAE individuals as early as possible, an early diagnosis is necessary. While not all disorders that can emerge as a consequence of PAE include physically visible deficits, all forms of FASD show neurobehavioral impairments. Thus, researchers have begun to assemble a neurobehavioral profile. With the help of this profile, diagnosis of all FASD forms should be facilitated.
Further research should focus on investigating the effects PAE has on children’s cognitive development in more depth. In addition, research should investigate the chances of alleviating symptoms in FASD individuals. The experiment on spatial working memory in section 5 exemplarily demonstrated the slower development of FASD individuals. However, it might be possible for them to develop similar skills if given more time. While preliminary research suggests that this is not the case, more studies need to be done to verify this possibility or investigate ways in which the development of FASD patients could be supported to match typically developing children and adolescents (Moore2021).
Additionally, more work is required to investigate the exact brain alterations in PAE individuals and how exactly they mediate impairments in the cognitive domain. Furthermore, most of the studies presented here are cross-sectional studies. Therefore, longitudinal studies are necessary to identify exact developmental patterns. Lastly, more research needs to be done to better understand which alcohol levels lead to impairments, as the exact relationship between PAE and FASD is not yet completely understood. Knowing critical levels of alcohol intake might help to prevent PAE in general.
By better understanding the impairments children with histories of prenatal alcohol exposure face in cognitive development, an earlier diagnosis is facilitated, leading to better support for the affected individuals. In the future, researchers might be able to find interventions that can reduce these impairments and improve the quality of life for PAE individuals.
References
Aragón, Alfredo S., Wendy O. Kalberg, David Buckley, Lindsey M. Barela-Scott, Barbara G. Tabachnick, and Philip A. May. 2008. “Neuropsychological Study of FASD in a Sample of American Indian Children: Processing Simple Versus Complex Information.” Alcoholism: Clinical and Experimental Research 32 (12): 2136–48.https://doi.org/10.1111/j.1530-0277.2008.00802.x.
Connor, Paul D., Paul D. Sampson, Fred L. Bookstein, Helen M. Barr, and Ann P. Streissgut. 2000. “Direct and Indirect Effects of Prenatal Alcohol Damage on Executive Function.” Developmental Neuropsychology 18 (3): 331–54. https://doi.org/10.1207/S1532694204Connor.
Fan, Jia, Sandra W. Jacobson, Paul A. Taylor, Christopher D. Molteno, Neil C. Dodge, Mark E. Stanton, Joseph L. Jacobson, and Ernesta M. Meintjes. 2016. “White Matter Deficits Mediate Effects of Prenatal Alcohol Exposure on Cognitive Development in Childhood.” Human Brain Mapping 37 (8): 2943–58.https://doi.org/10.1002/hbm.23218.
Green, C. R., A. M. Mihic, S. M. Nikkel, B. C. Stade, C. Rasmussen, D. P. Munoz, and J. N. Reynolds. 2009. “Executive Function Deficits in Children with Fetal Alcohol Spectrum Disorders (FASD) Measured Using the Cambridge Neuropsychological Tests Automated Battery (CANTAB).” Journal of Child Psychology and Psychiatry 50 (6): 688–97.https://doi.org/10.1111/j.1469-7610.2008.01990.x.
Howell, K., M. E. Lynch, K. A. Platzman, G. Smith, and C. D. Coles. 2005. “Prenatal Alcohol Exposure and Ability, Academic Achievement, and School Functioning in Adolescence: A Longitudinal Follow-up.” Journal of Pediatric Psychology 31 (1): 116–26.https://doi.org/10.1093/jpepsy/jsj029.
Kully-Martens, K., J. Pei, J. Job, and C. Rasmussen. 2012. “Source Monitoring in Children with and Without Fetal Alcohol Spectrum Disorders.” Journal of Pediatric Psychology 37 (7): 725–35.https://doi.org/10.1093/jpepsy/jsr123.
Lee, K. T., S. N. Mattson, and E. P. Riley. 2004. “Classifying Children with Heavy Prenatal Alcohol Exposure Using Measures of Attention.” Journal of The International Neuropsychological Society 10 (2): 271–77.https://doi.org/10.1017/s1355617704102142.
Mattson, S. N., G. A. Bernes, and L. R. Doyle. 2019. “Fetal Alcohol Spectrum Disorders: A Review of the Neurobehavioral Deficits Associated with Prenatal Alcohol Exposure.” Alcoholism: Clinical and Experimental Research. 43 (6): 1046–62.https://doi.org/10.1111/acer.14040.
Moore, E. M., L. Glass, M. A. Infante, C. D. Coles, J. A. Kable, K. L. Jones, E. P. Riley, and S. N. Mattson. 2021. “Cross‐sectional Analysis of Spatial Working Memory Development in Children with Histories of Heavy Prenatal Alcohol Exposure.” Alcoholism: Clinical and Experimental Research 45 (1): 215–23.https://doi.org/10.1111/acer.14506.
Moore, E. M., R. Migliorini, M. A. Infante, and E. P. Riley. 2014. “Fetal Alcohol Spectrum Disorders: Recent Neuroimaging Findings.” Current Developmental Disorders Reports 1 (3): 161–72.https://doi.org/10.1007/s40474-014-0020-8.
Riley, E. P., M. A. Infante, and K. S. Warren. 2011. “Fetal Alcohol Spectrum Disorders: An Overview.” Neuropsychology Review 21 (2): 73–80.https://doi.org/10.1007/s11065-011-9166-x
Research Question:
How is cognitive development of children with histories of prenatal alcohol exposure affected?
